Cabotegravir is an HIV-1 integrase strand transfer inhibitor (INSTI) developed by ViiV Healthcare. As a significant advancement in the field of antiretroviral therapy, Cabotegravir offers two dosage forms: oral tablets and long-acting injectables, bringing new possibilities for the treatment and prevention of HIV-1 infection.
Indications of Cabotegravir
Treatment of HIV-1 Infection
Cabotegravir, in combination with EDURANT (rilpivirine), is indicated for the short-term treatment of HIV-1 infection in adult patients and adolescent patients aged 12 years and older who weigh at least 35 kg.
The specific eligible population must meet the following criteria:
Be in a virologically suppressed state (HIV-1 RNA < 50 copies/mL).
Be on a stable antiretroviral treatment regimen.
Have no history of treatment failure.
Have no known or suspected resistance to cabotegravir or rilpivirine.
HIV-1 Pre-Exposure Prophylaxis (PrEP)
Cabotegravir is also approved for short-term pre-exposure prophylaxis to reduce the risk of acquiring HIV-1 infection through sexual transmission. It is indicated for adult and adolescent patients who weigh at least 35 kg. A negative HIV-1 test result must be confirmed before use.
Specific applications include:
Used as oral lead-in therapy to assess tolerance to cabotegravir, preparing for subsequent use of APRETUDE (cabotegravir long-acting injectable suspension).
Used as oral PrEP medication for patients who may miss scheduled injection doses of APRETUDE.
Specifications and Properties of Cabotegravir
Dosage Form and Specifications
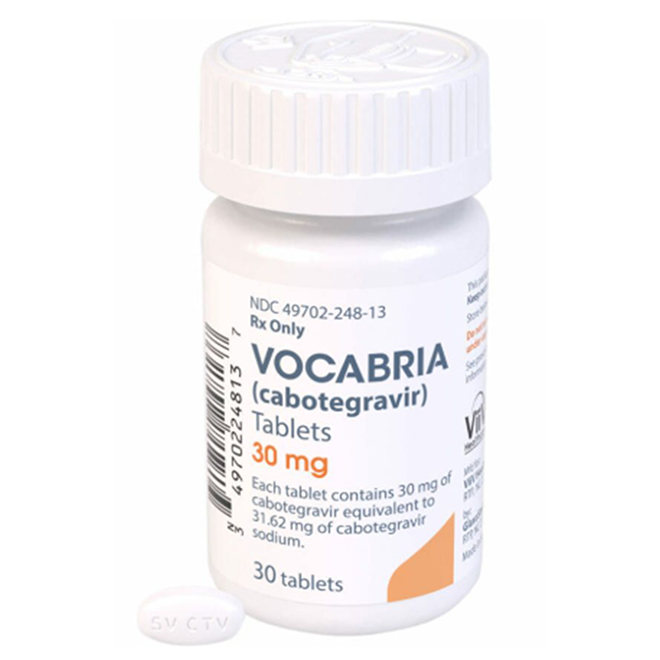
White to off-white film-coated tablets.
Oval, biconvex tablets.
Specification: 30 mg per tablet.
One side of the tablet is engraved with "SVCTV" and the other side with "30" (both in intaglio).
Storage Methods for Cabotegravir
Temperature Control
Recommended storage at 20°C–25°C.
Short-term exposure to a temperature range of 15°C–30°C is permitted.
Humidity Control
Should be stored in the original packaging to prevent moisture absorption.
Keep away from humid environments such as bathrooms.
Light Protection
Must be stored away from light to avoid direct sunlight.
It is recommended to keep the tablets in the original aluminum-plastic blister packaging until administration.
Safe Storage
Store out of the reach of children and pets.
Do not store in locations with large temperature fluctuations, such as the glove compartment of a vehicle.
Disposal of Expired Medication
Pay attention to checking the expiration date of the medication.
Expired medication should be recycled and disposed of through formal channels.

Free Inquiry

















