
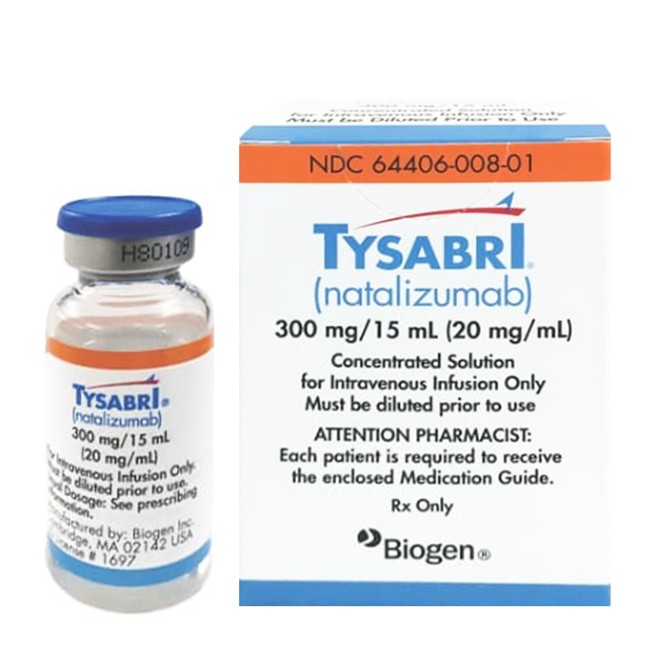


Natalizumab(Tysabri)
Reminder: The outer packaging is for reference only, please purchase and use under the guidance of a pharmacist. For read by medical and pharmaceutical professionals only.
Tysabri(Natalizumab) Instructions:Uses,Dosage, Side Effects
Unopened vials of Tysabri (Natalizumab) should be stored refrigerated at 2°C–8°C, protected from light, and must not be frozen or shaken.
Diluted solution: Store refrigerated for no more than 48 hours and do not freeze.
It is indicated for monotherapy of relapsing multiple sclerosis in adults, as well as induction and maintenance therapy of moderately to severely active Crohn's disease. Patients with Crohn's disease (CD) should not use it in combination with immunosuppressants or TNF-α inhibitors.
The injection is available in a concentration of 300mg/15mL (20mg/mL), presenting as a colorless to slightly opalescent solution, which requires dilution before intravenous infusion. The recommended dose is 300mg per administration, given intravenously once every 4 weeks, with the infusion time lasting approximately 1 hour. Intravenous push or rapid injection is strictly prohibited.





- Core products
- Antitumor drugs
- General drug
- Antiviral drugs
- Biological agents
- haiousales@gmail.com












