Avelumab (Bavencio) is a humanized monoclonal antibody targeting PD-L1, approved globally for the treatment of solid tumors including metastatic Merkel cell carcinoma, locally advanced or metastatic urothelial carcinoma, and advanced renal cell carcinoma. As a special medication requiring strict medical supervision, the accessibility of procurement channels, standardized use, and authenticity verification have a decisive impact on treatment outcomes.
What are the Procurement Channels for Avelumab (Bavencio)?
Overseas Procurement
Patients may consult and purchase avelumab at hospital pharmacies or licensed drugstores in countries/regions where the medication is approved for marketing.
Due to potential price variations influenced by regional differences, exchange rate fluctuations, and other factors, patients should conduct budget planning prior to purchase.
Procurement through Medical Service Institutions
Patients may consult domestic overseas medical service institutions collaborating with international pharmacies or pharmaceutical companies.
These institutions typically provide legal import channels and offer professional consultation and guidance.
Precautions for the Use of Avelumab (Bavencio)
Pre-Infusion Prophylactic Medication Specifications
For the first treatment, premedication with antihistamines and acetaminophen is required, and this precaution should be maintained for at least the first four infusions.
Healthcare providers must closely monitor for infusion-related reactions, including clinical manifestations such as fever (26% incidence), chills, flushing, and hypotension.
Special Management Plan for Combined Medication
Patients with advanced renal cell carcinoma should receive avelumab in combination with axitinib (5 mg orally twice daily), and special attention should be paid to the risk of synergistic toxicity.
Contraindications and Warnings for Special Populations
Pregnant Women: The drug’s mechanism may cause immune-mediated fetal rejection. Effective contraceptive measures must be used during treatment and for at least 1 month after discontinuation.
Graded Management of Immune-Related Adverse Reactions
Immune-Mediated Pneumonitis (incidence 1.1%): Manifested as cough, chest pain, and shortness of breath.
Immune-Mediated Hepatitis (incidence 1.1%): Monitor liver enzyme indicators (ALT/AST).
Immune-Mediated Endocrine Disorders: Including thyroid dysfunction (5%) and adrenal insufficiency (0.6%).
Cardiac Adverse Events (MACE incidence 7%): Including myocardial infarction (2.8%) and heart failure (1.8%).
Authenticity Verification of Avelumab (Bavencio)
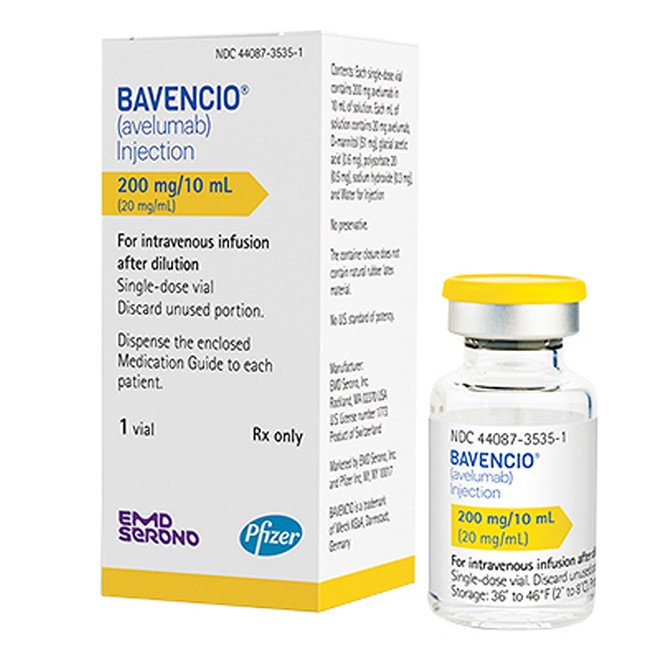
Packaging Specification Verification Standards
Authentic products are supplied as single-dose vials with a specification of 200 mg/10 mL (20 mg/mL).
The solution must meet the characteristic standard of "clear, colorless to slightly yellow."
The packaging should indicate the manufacturer (EMD Serono, Inc.) and the latest revision date.
Cold Chain Management and Traceability Verification
The entire circulation process of the drug must be maintained in a cold chain environment of 2°C-8°C; any temperature deviation will cause protein denaturation and inactivation.
Correlative Analysis of Clinical Monitoring Indicators
Baseline Assessment: Liver function (ALT/AST), renal function (creatinine), and thyroid function.
Pre-Infusion Inspection: Visually inspect the solution for clarity; do not use if turbidity, particles, or discoloration is observed.

Free Inquiry

















