Ivosidenib (Tibsovo) is an important targeted therapy drug against IDH1 mutations, demonstrating significant efficacy in diseases such as acute myeloid leukemia and cholangiocarcinoma. For patients in need of this medication, understanding legitimate purchase channels, mastering medication precautions, and learning to identify drug authenticity are key steps to ensure effective treatment.
What Are the Purchase Channels for Ivosidenib (Tibsovo)?
Overseas Purchase
Patients may choose to consult and purchase the drug at hospital pharmacies or licensed pharmacies in countries/regions where ivosidenib has been approved for marketing.
As drug prices may be affected by factors such as regional differences and exchange rate fluctuations, patients need to make a budget and plan in advance before purchasing.
Purchase via Medical Service Institutions
Patients can consult domestic overseas medical service institutions that cooperate with international pharmacies or pharmaceutical companies.
These institutions usually provide legal import channels, along with professional consultation and guidance services.
Precautions for the Use of Ivosidenib (Tibsovo)
Professional Testing and Consultation
Prior to considering the use of ivosidenib, professional IDH1 mutation testing must be performed to confirm the presence of applicable mutation types.
Common IDH1 mutations include R132C, R132H, R132G, etc.
Patients should undergo relevant testing and evaluation under the guidance of a physician.
Drug Interactions
Special attention should be paid to drug interactions during the administration of ivosidenib.
Concomitant use with strong or moderate CYP3A4 inhibitors may increase the plasma concentration of ivosidenib, thereby potentially raising the risk of QT interval prolongation.
Patients must inform their physicians of all medications they are currently taking, including prescription drugs, over-the-counter drugs, vitamins, and herbal supplements.
Medication in Special Populations
For pregnant women, lactating women, and patients with hepatic or renal insufficiency, consultation with a physician is mandatory before use to assess the risks of medication.
The drug may cause fetal harm; therefore, effective contraceptive measures should be adopted during the treatment period.
Lactating women should discontinue breastfeeding during treatment and for 1 month after the last dose.
Storage and Administration Specifications
Ivosidenib tablets should be swallowed whole; they must not be split, crushed, or chewed.
Store the drug in a dry place with the bottle cap tightly closed, and do not remove the desiccant canister inside the bottle.
Identification of Authentic Ivosidenib (Tibsovo)
Packaging and Label Inspection
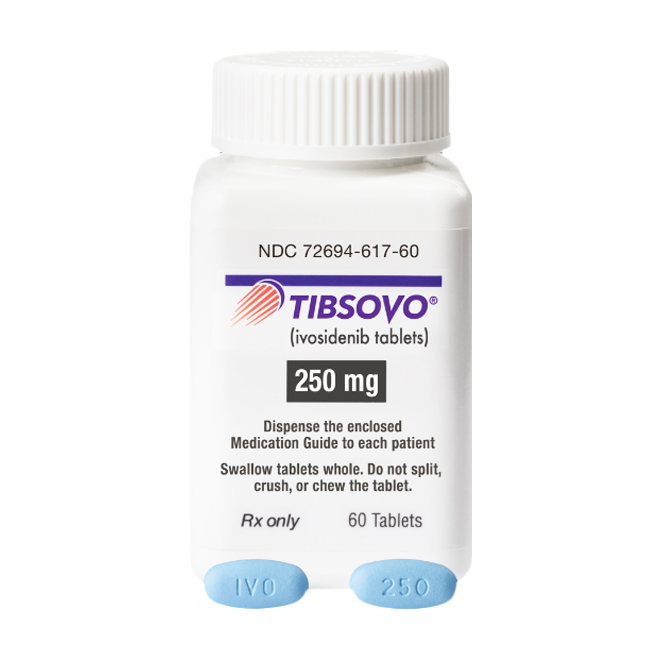
The packaging of authentic ivosidenib should bear clear information, including drug name, dosage form, batch number, and expiration date.
Authentic tablets are blue, oval, film-coated tablets, engraved with "IVO" on one side and "250" on the other side, corresponding to the 250 mg strength.
Carefully check whether the packaging is intact, whether the label printing is clear, and whether the color is uniform.
Verification via Official Channels
Check the drug's approval number and manufacturer information through official channels such as the National Medical Products Administration to ensure consistency with the authentic product.

Free Inquiry

















