With the advancement of medical technology, elacestrant (Orserdu), a targeted drug for breast cancer with specific gene mutations, has brought new treatment options for patients. As the first oral selective estrogen receptor degrader (SERD), it is especially indicated for patients with ESR1-mutant, ER+/HER2- advanced or metastatic breast cancer.
What Are the Purchase Channels for Elacestrant (Orserdu)?
Overseas Purchase
Patients may opt to consult and purchase the drug at hospital pharmacies or licensed pharmacies in countries or regions where elacestrant has been approved for marketing.
Since drug prices may be affected by factors such as regional differences and exchange rate fluctuations, patients need to make a budget and plan in advance before purchasing.
Purchase via Medical Service Institutions
Patients can consult domestic overseas medical service institutions that cooperate with international pharmacies or pharmaceutical companies.
These institutions can usually provide legal import channels, as well as professional consultation and guidance.
Important Precautions for Purchasing and Using Elacestrant (Orserdu)
Mandatory Genetic Testing
Before using elacestrant, it is imperative to confirm the presence of ESR1 mutation in patients through FDA-approved testing methods.
Only patients with ESR1 mutation can derive significant benefits, while the efficacy in non-mutant populations is limited. This is a critical prerequisite for ensuring treatment effectiveness.
Ensure Complete Prescriptions and Qualification Documents
Regardless of the purchase channel chosen, patients should request comprehensive drug traceability information and official invoices.
Meanwhile, a doctor-issued prescription must be provided to ensure that medication administration complies with medical standards.
Drug Interactions
Elacestrant interacts with strong or moderate CYP3A4 inducers and inhibitors, and concomitant use with these drugs should be avoided.
Elacestrant is an inhibitor of P-gp and BCRP; dosage adjustment is required when co-administered with substrates of these transporters.
Medication Guidelines for Special Populations
For patients with hepatic impairment, elacestrant should be avoided in those with severe hepatic impairment, and dosage adjustment is necessary for those with moderate hepatic impairment.
Women of childbearing potential and male patients must use effective contraceptive measures during treatment and for one week after discontinuation, as the drug may cause fetal harm.
Identification of Authentic Elacestrant (Orserdu)
Inspect Drug Packaging and Labeling
The packaging of genuine elacestrant should have clear printing and uniform color, with complete labeling information affixed to the bottle.
The label should include details such as drug name, specification, batch number, expiration date, and manufacturer.
The bottle cap should be designed with anti-counterfeiting features or a child-resistant lock.
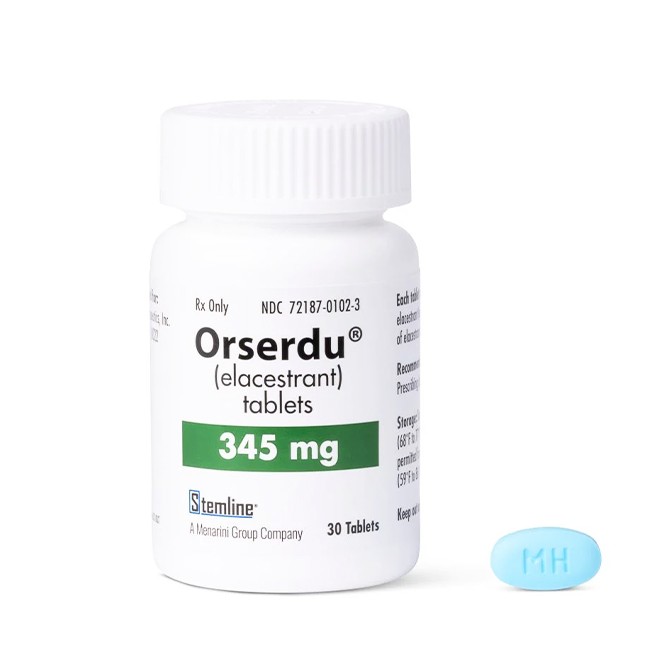
Verify Drug Appearance and Markings
Elacestrant tablets are light blue. The 345mg tablet is oval-shaped and engraved with the marking "MH".
The 86mg tablet is round and engraved with the marking "ME".
If the tablet’s color, shape, or marking is blurred or inconsistent with the above descriptions, it is highly likely to be a counterfeit product.

Free Inquiry

















