Elacestrant (Orserdu) is a novel estrogen receptor antagonist that plays an important role in the treatment of ER-positive, HER2-negative, ESR1-mutated advanced or metastatic breast cancer.
What Are the Purchase Channels for Elacestrant (Orserdu)?
Overseas Purchase
Patients may choose to consult and purchase elacestrant at hospital pharmacies or formal drugstores in countries or regions where the medication has been launched.
Since drug prices may be affected by factors such as regional differences and exchange rate fluctuations, patients need to make a budget and plan in advance before purchasing.
Purchase Through Medical Service Institutions
Patients may consult domestic overseas medical service institutions that cooperate with international pharmacies or pharmaceutical companies.
These institutions can usually provide legal import channels and offer professional consultation and guidance.
Precautions for Purchasing Elacestrant (Orserdu)
Strict Medical Evaluation
According to the drug instructions, the ESR1 mutation status must be confirmed through an FDA-approved test before using elacestrant.
This medication is specifically indicated for postmenopausal women or adult men with ER-positive, HER2-negative, ESR1-mutated advanced or metastatic breast cancer, who have experienced disease progression after receiving at least one line of endocrine therapy.
Professional Medication Guidance
The recommended dose of elacestrant is 345 mg orally once daily, which should be taken with meals to reduce symptoms of nausea and vomiting.
The tablets should be swallowed whole and must not be chewed, crushed, or split. Do not take the tablets if they are found to be broken, cracked, or damaged in appearance.
Medication Warnings for Special Populations
Patients with severe hepatic impairment should avoid using elacestrant.
Patients with moderate hepatic impairment need to reduce the dose to 258 mg once daily. Patients with mild hepatic impairment do not require dose adjustment.
Authentication of Elacestrant (Orserdu) (Genuine vs. Counterfeit)
Identification of Appearance Characteristics
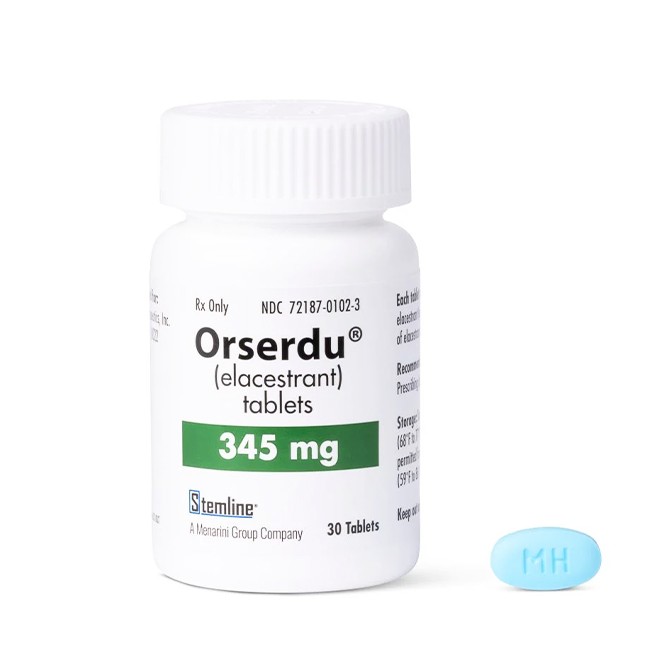
Genuine elacestrant tablets have specific appearance features:
The 345 mg tablet is light blue, unscored, oval-shaped, film-coated, and biconvex, with "MH" printed on one side and blank on the other.
The 86 mg tablet is light blue, unscored, round-shaped, film-coated, and biconvex, with "ME" printed on one side and blank on the other.
Inspection of Packaging Integrity
Check whether the drug packaging is intact; the bottle cap should be equipped with a child-resistant lock.
The packaging should clearly indicate information such as the drug name, strength, batch number, and expiration date.
Verification of Official Information
The FDA-approved label for elacestrant in the United States is manufactured and distributed by Stemline Therapeutics, Inc.
The packaging should indicate complete storage condition information: 20°C to 25°C, with a permitted deviation between 15°C and 30°C.
Any details inconsistent with the official description should arouse vigilance.

Free Inquiry

















